The human brain is understudied when it comes to its cellular, subcellular, vascular, and neural micro-organization. This lack of data has a huge impact on our understanding of many currently incurable neurological, neurovascular, and psychiatric disorders. The emerging field of large-scale electron microscopy to map the fine structure of brains, known as connectomics, would be more useful if it could be applied to human brain samples. Unfortunately, autopsied brain samples are not ideal for connectomics, given the poor preservation of microstructure after death. Additionally, complete local network analysis requires preservation of contiguous large volumes of brain is required. In animals, it has been possible to acquire samples immediately after death by perfusing the animal with chemical fixatives via the major blood vessels leaving the heart. This approach is not possible in human patients.
In this recently published work, we describe a method where small samples of human medical biopsies made available at the time of neurosurgery are rapidly preserved and then stained with heavy metals and embedded in a hard resin for connectomic studies. These patients are undergoing brain surgery to remove sites that initiate epileptic seizures or for implantation of electrical leads for deep brain stimulation procedures that are used to treat Parkinson’s disease and certain psychiatric disorders. The samples collected would otherwise have been discarded.
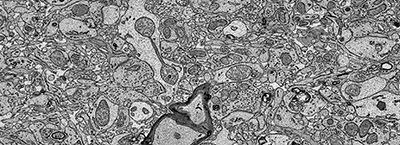
Glimpse of a freshly preserved biopsy of human cerebral cortex
The devised immersion fixation and heavy metal staining method provides excellent preservation of volumes that are 8-37 mm3 and large enough to trace neural circuits over long distances. The hallmark of this technique is that not only it preserves the finest details of brain’s ultrastructure but also preserves the extracellular space between brain cells which disappears with traditional fixation techniques. Neurons as well as the supporting glial cells could be easily traced in these samples with both manually and automatically using recently developed machine learning algorithms. This rapid preservation also reveals labile microstructures such as fusing vesicles and invaginating cellular processes at synapses and in the vasculature.
Ultimately, we hope that this high-quality preservation of human brain samples will add to our comprehension of brain disorders where current lower resolution methods have not found evidence of abnormalities.
by Neha Karlupia and Jeff Lichtman
 (PDF)
(PDF)