A fundamental question in developmental biology is how multi-potent cells choose a fate. While we know a lot about how cell states are maintained through growing amounts of large scale data, we know little about how cells make transitions between these states.
We used embryonic stem cells as a model system to understand how cells decide on their fate. We studied how these pluripotent cells chose between the two germ layer fates (mesendoderm giving rise to muscle, bone and gut; and neural ectoderm giving rise to neurons and skin).
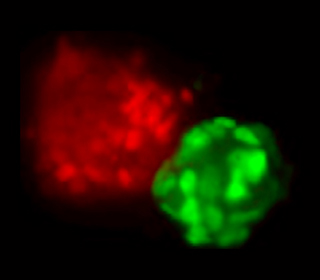
The image, to your left, shows a field of nuclei of cells that started off as pluripotent stem cells. After exposure to differentiation signals, some of these cells chose the mesendodermal fate (red) and others, the neural ectodermal fate (green).
We made two key assumptions about the circuit that controls this cell fate choice: (1) the circuit proteins have to be expressed in the cell before it commits to a germ layer and (2) the changes in levels of these proteins as the cell makes a choice have to reflect the decision.
We found that of all the DNA binding proteins in embryonic stem cells, the levels of only three proteins are modulated in a germ-layer fate choice dependent manner. Further, this modulation occurs continuously and well before the cells commit to a fate. Surprisingly, we found that two of these proteins, Oct4 and Sox2, which are key regulators that maintain the cells in the pluripotent state also mediate germ layer fate choice. Cells in which Oct4 is up regulated and Sox2 down regulated, inhibit neural ectodermal specific genes while promoting mesendodermal gene expression. Conversely, cells with increasing Sox2 and decreasing Oct4 levels promote neural ectodermal differentiation while inhibiting mesendodermal differentiation.
Such lineage specific roles of the key pluripotency factors allow the cell to integrate external signal and make a fate choice. In particular, we found that this design allows cells to remain undecided in the face of conflicting signals.
Read more in Cell
(Matt Thomson, Siyuan John Liu ’11, Ling Nan Zou, Zack Smith and Alexander Meissner (SCRB) are the co-authors of this paper)
[June 10h, 2011]